Patrick Bet-David explains how the FDA has officially approved CRISPR gene editing as a treatment for Sickle Cell Disease, marking a historic milestone in medical science.
The science behind CRISPR-Cas9, a revolutionary gene-editing tool has transformed biomedical research. Understand how it can precisely alter DNA to correct genetic defects, potentially treating a range of illnesses. As CRISPR technology advances, so does the debate over its ethical use.
Learn the benefits of becoming a Valuetainment Member and subscribe today!
PBD also discusses the controversial topic of designer babies, examining the moral implications and potential societal impacts of gene editing for non-therapeutic purposes.
On Dec. 8th, the Food and Drug Administration (FDA) approved two gene-editing drugs which will be used to treat sickle-cell disease. The treatments, Casgevy and Lyfgenia, are both cell-based gene therapies, but the former is the first to have been approved by the FDA that utilizes a new type of genome editing technology called CRISPR.
Casgevy can be used to treat patients 12 years or older who experience recurrent vaso-occlusive crises. CRISPR is a gene-editing technology that transforms genomes by modifying patients’ hematopoietic (blood) stem cells. It splices DNA in targeted areas and has the power to remove, add, or replace specific strands.
This means that it can hypothetically do this to any part of a genome. It could potentially allow parents to change the color of hair, height, eye color, body size, and so on.
According to one medical study, CRISPR technology can potentially be used to treat different types of cancer, blood disorders, blindness, AIDS, cystic fibrosis, muscular dystrophy, Huntington’s disease, and COVID-19.
It was reported in 2020 by CNBC via U.S. intelligence that China has been conducting tests on human subjects in the People’s Liberation Army with the intent of creating soldiers with “biologically enhanced capabilities.”
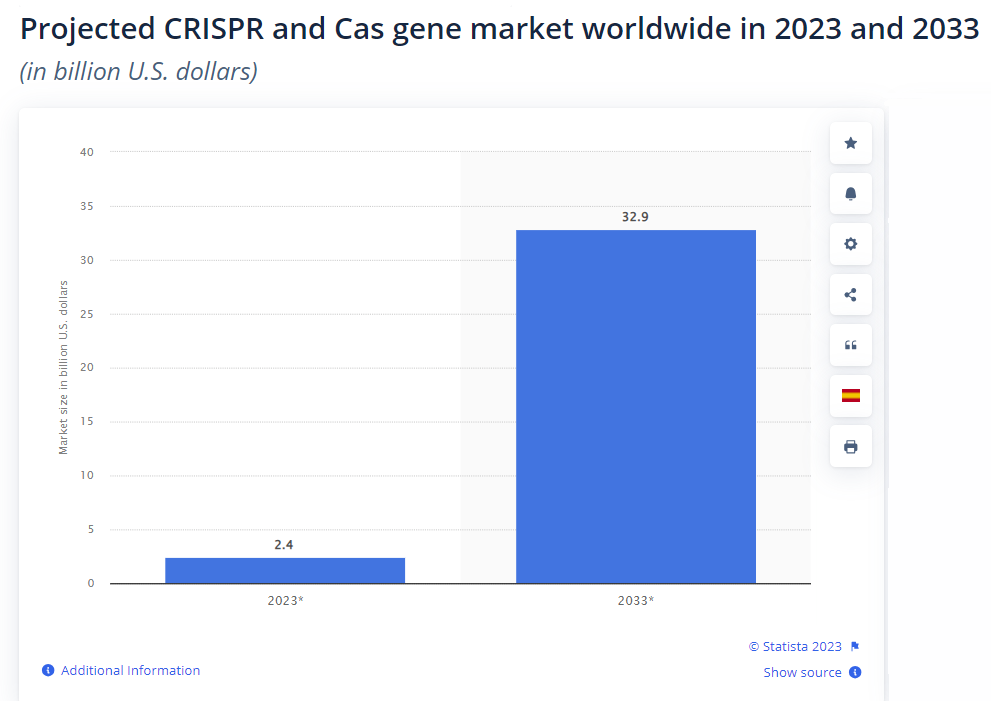
According to Statista, the market size for gene-editing technology in 2023 is roughly $2.4 billion. In 10 years, market speculators predict it will climb to $33 billion.
CRISPR can also be used to edit DNA in plants and animals, altering specific DNA sequences toward greater produce and livestock yields — or something more nefarious.
Watch the rest of the video to hear what else could come from CRISPR.


















Add comment