Elon Musk said late Monday that the first human being has received a Neuralink implant, and the “brain-computer interface” technology is so far showing “promising” signs.
“The first human received an implant from @Neuralink yesterday and is recovering well,” Musk announced via X. “Initial results show promising neuron spike detection,” he added, meaning that it is detecting signals from neurons and reporting them to external devices.
The first human received an implant from @Neuralink yesterday and is recovering well.
Initial results show promising neuron spike detection.
— Elon Musk (@elonmusk) January 29, 2024
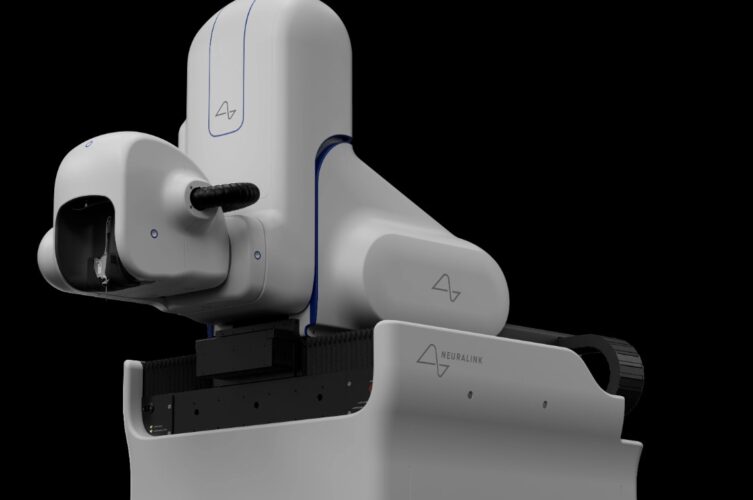
By “recovering well,” Musk was referencing the subject’s surgery, as the N1 implant involves a delicate implanting procedure using a “surgical robot” created by Neuralink. The robot inserts some 1,000 electrodes across 64 neuron-detecting threads—so fine that they cannot be manipulated by human hands—using its five cameras and a needle operated in linear motion.
Learn the benefits of becoming a Valuetainment Member and subscribe today!
The device is inserted into a “biocompatible enclosure” in the brain designed to operate from within a human body. While it runs off a battery, it is charged wirelessly through a small charger. The chips inside the N1 detect and process neural signals which it then delivers to a software system that makes sense of the data and transforms it into commands for the electronic devices (like computers and phones) it is in communication with.
Elon also announced that the first Neuralink product will be called “Telepathy.” He said it will give users control of a computer or phone “just by thinking.”
The computers and phones then send orders to other things in the house: lights, windows, kitchen appliances, and anything else that can be controlled remotely through apps.
“Initial users will be those who have lost the use of their limbs,” Musk specified. “Imagine if Stephen Hawking could communicate faster than a speed typist or auctioneer. That is the goal.”
Enables control of your phone or computer, and through them almost any device, just by thinking.
Initial users will be those who have lost the use of their limbs.
Imagine if Stephen Hawking could communicate faster than a speed typist or auctioneer. That is the goal.
— Elon Musk (@elonmusk) January 30, 2024
In September, Neuralink had announced it was beginning its first human clinical trials in a study called Precise Robotically Implanted Brain-Computer Interface, or PRIME.
Related: Elon Musk’s Neuralink Begins Recruiting for its First Clinical Trial
The Food and Drug Administration (FDA) approved the human implant procedure in May. While the patient of this first trial has not been named, Neuralink had said in September that it was recruiting people who suffer from quadriplegia due to cervical spinal cord injury as well as amyotrophic lateral sclerosis (ALS). It also stipulated that participants must be 22 years old or older and have a reliable caretaker. Furthermore, it said it could not accept people who have a history of seizures, have an active implanted device, require MRIs for a medical condition, or are receiving transcranial magnetic stimulation (TMS) treatment.
 Shane Devine is a writer covering politics, economics, and culture for Valuetainment. Follow Shane on X (Twitter).
Shane Devine is a writer covering politics, economics, and culture for Valuetainment. Follow Shane on X (Twitter).

















Add comment