Pharmaceutical companies have a very unique business model. Brand name drug companies use patents to prevent the production of generic drugs.
- Current law provides brand pharmaceutical companies with 12 years of guaranteed market exclusivity for biologics and 20 years for each patent.
- During the period of patent and marketing exclusivity, brand drugs are priced and sold free from competition.
Strategic Patenting by Pharmaceutical Companies – Should Competition Law Intervene? – PMC (nih.gov)
- According to I-MAK, the top 12 brand drugs on the market are protected by a total of 848 patents (71 per drug) providing an average of 38 years without generic competition.
- The world’s top-selling brand drug, Humira, treats arthritis and other chronic conditions. On the market since 2002, 132 patents block competition for up to 39 years.
Evergreening: This practice involves making slight modifications to an existing drug, such as changing its formulation, dosage, or delivery method, and then patenting the new version. The company then encourages healthcare providers to prescribe the new version, even though the original drug may still be effective.
Subscribe to PBD’s YouTube channel today for every episode LIVE or On Demand!
Patent term extension: In some jurisdictions, companies can apply for a patent term extension if they can demonstrate that they have invested significant time and resources in developing the drug. This extension is typically granted to compensate for the time spent in research and regulatory approval processes.
Data exclusivity: Pharmaceutical companies often receive a period of data exclusivity, during which they have exclusive rights to the clinical trial data they’ve generated. This can delay the entry of generic drugs into the market, as other manufacturers must wait until the exclusivity period expires to access the data and develop their own versions.
Patent clustering: Companies may file multiple patents around a single drug, covering various aspects such as manufacturing processes, formulations and even potential uses. This can make it more difficult for generic manufacturers to enter the market without infringing on any of the existing patents.
Pay-for-delay agreements: Sometimes, pharmaceutical companies enter into agreements with generic drug manufacturers to delay the introduction of a generic version of a drug. In these deals, the brand-name drug manufacturer compensates the generic manufacturer for not launching their product for a specified period.
Orphan drug exclusivity: In some cases, drugs developed to treat rare diseases (orphan drugs) receive extended market exclusivity to encourage companies to invest in research and development for these conditions.
Legal challenges: Pharmaceutical companies may engage in litigation to enforce their patents or challenge the validity of generic competition.
What Happens When Drug Patents Expire?
- Data shows prescription drug costs decline by 90% in some cases when patents expire and competition enters the market.
- It is estimated that the generic entry typically leads to, on average, an 80 percent market share loss.
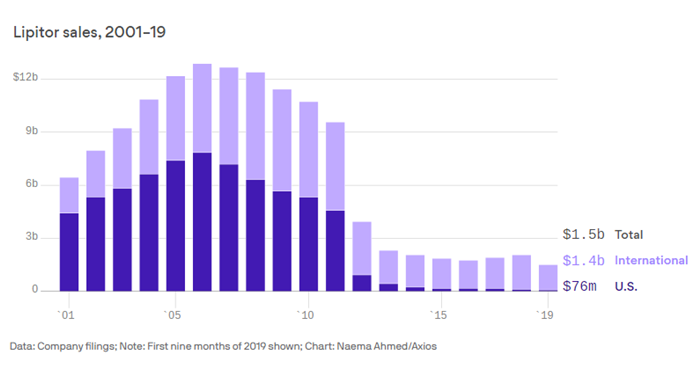
Lipitor — Pfizer’s cholesterol-lowering medication — (Patent Expired 2011)
Cholesterol drug Lipitor is still generating billions for Pfizer (axios.com)
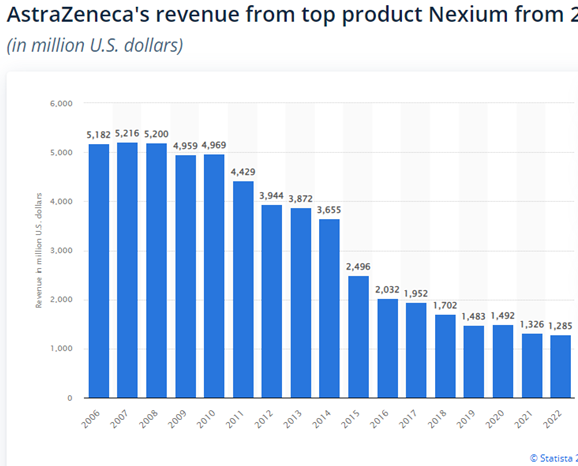
Nexium — a drug for gastroesophageal reflux disease — (Patent expired in 2014)
AstraZeneca Nexium revenue top product 2006-2022 | Statista
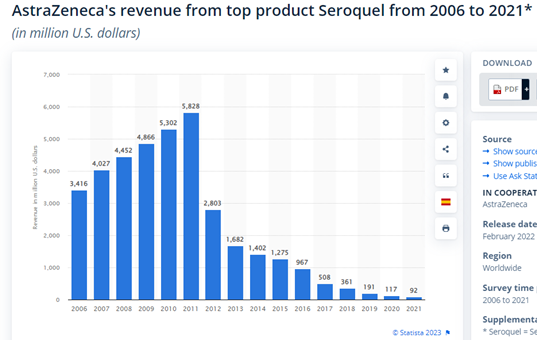
Seroquel — for schizophrenia and bipolar disorder — Patent Expired in 2012
AstraZeneca Seroquel revenue top product 2021 | Statista
The History of U.S. Pharmaceutical Patent Laws
The Patent Act of 1790: This was the first patent statute enacted in the United States. Established the basic framework for granting patents and protecting inventors’ rights, which later extended to the pharmaceutical industry.
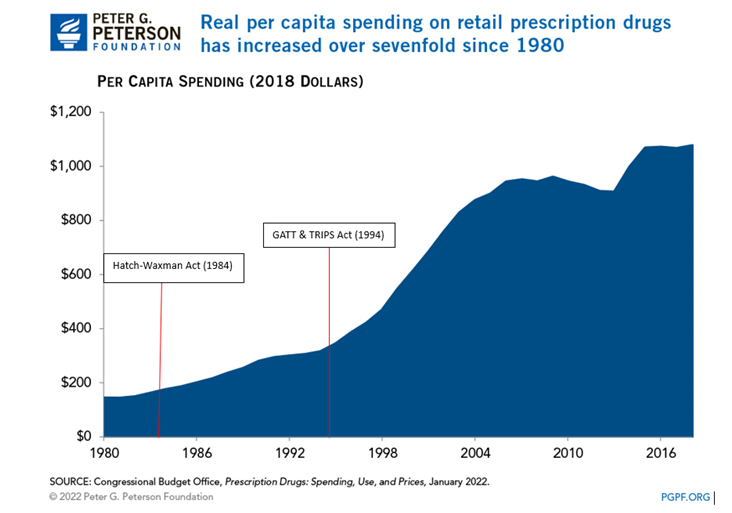
Hatch-Waxman Act (1984): Allowed brand names up to 14 years of exclusive patents rights after FDA approval.
The generic drug industry supported the Hatch-Waxman Act of 1984 because it was sold as a pathway for generic drugs to come to market more quickly and at a lower cost.
GATT and TRIPS (1994): Extended the minimum patent term to 20 years from filing. This was implemented in U.S. law in 1995.
How Have Prescription Drug Prices Changed Over Time? (pgpf.org)
Arguments for Pharmaceutical Patents
- Incentivizing Innovation
- Promoting Drug Access and Availability
- Protecting Intellectual Property Rights
Arguments against Pharmaceutical Patents
- High Drug Prices and Access Barrier
- Monopolistic Practices and Lack of Competition
- Limited Innovation and Repurposing
- Publicly Funded Research and Development
- Ethical Considerations
Who can stop this?
YOU, ME, CONGRESS, and the PRESIDENT!


















Add comment