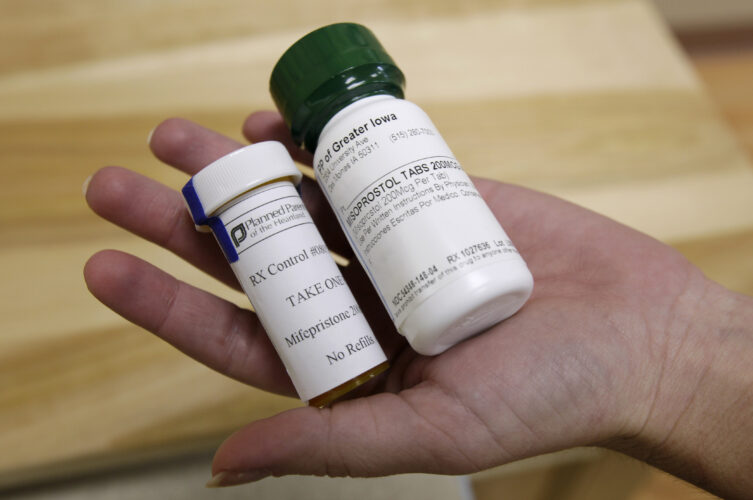
A federal appeals court has allowed the abortion pill Mifepristone to remain on the U.S. market for now but added major restrictions on accessing the medication.
The U.S. 5th Circuit Court of Appeals late Wednesday blocked U.S. District Judge Matthew Kacsmaryk’s order, suspending the Food and Drug Administration’s approval of Mifepristone. The three-judge panel voted 2-1 in favor of re-imposing restrictions on mifepristone, potentially taking it up to the Supreme Court.
The court temporarily rolled back on some of the major changes the FDA had implemented over the years to make the medical abortion pill easier to access. This order bars mail delivery of the pill, meaning patients will now have to obtain a prescription from their doctor after undergoing several examinations while taking the medication.
Major side effects have been recorded from women seeking an abortion using mifepristone including, detrimental fevers, severe pelvic pain, dizziness, nausea or vomiting, accelerated heart rates, fever, chills, fever and overall weakness.
The court also cited a warning to the FDA that surgery might be necessary if the use of Mifepristone results in an incomplete abortion. At present, the agency requires patients to sign a form indicating that the drug may not work in 2 to 7 out of 100 women who take the pill.
Patients are instructed to contact their health care provider if they have a fever of or over 100.4 degrees for more than 4 hours, heavy bleeding or severe stomach discomfort.
“FDA thus cannot deny that serious complications from mifepristone are certainly impending,” the judges wrote in it order. “Those complications are right there on the Patient Agreement Form that FDA itself approved and that Danco requires every mifepristone user to sign.”

















Add comment